- Accueil
- mcv4
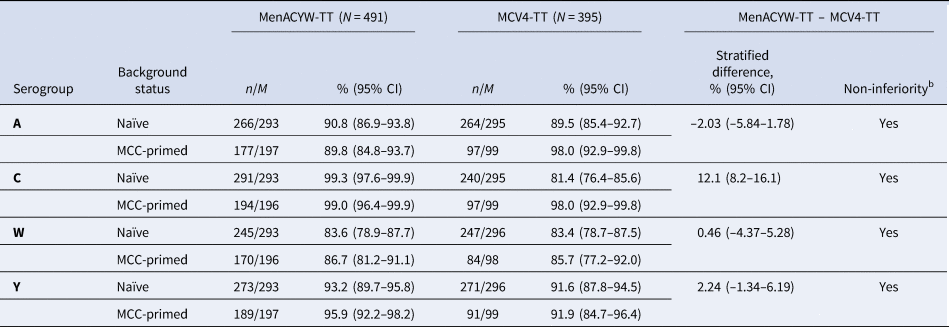
- Immunogenicity and safety of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine (MenACYW-TT) vs. a licensed quadrivalent meningococcal tetanus toxoid-conjugate vaccine in meningococcal vaccine-naïve and meningococcal C conjugate vaccine
Immunogenicity and safety of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine (MenACYW-TT) vs. a licensed quadrivalent meningococcal tetanus toxoid-conjugate vaccine in meningococcal vaccine-naïve and meningococcal C conjugate vaccine
4.5 (689) · € 26.50 · En Stock

PDF] Meningococcal Vaccination: Recommendations of the Advisory Committee on Immunization Practices, United States, 2020

Meningococcal vaccine - Wikipedia
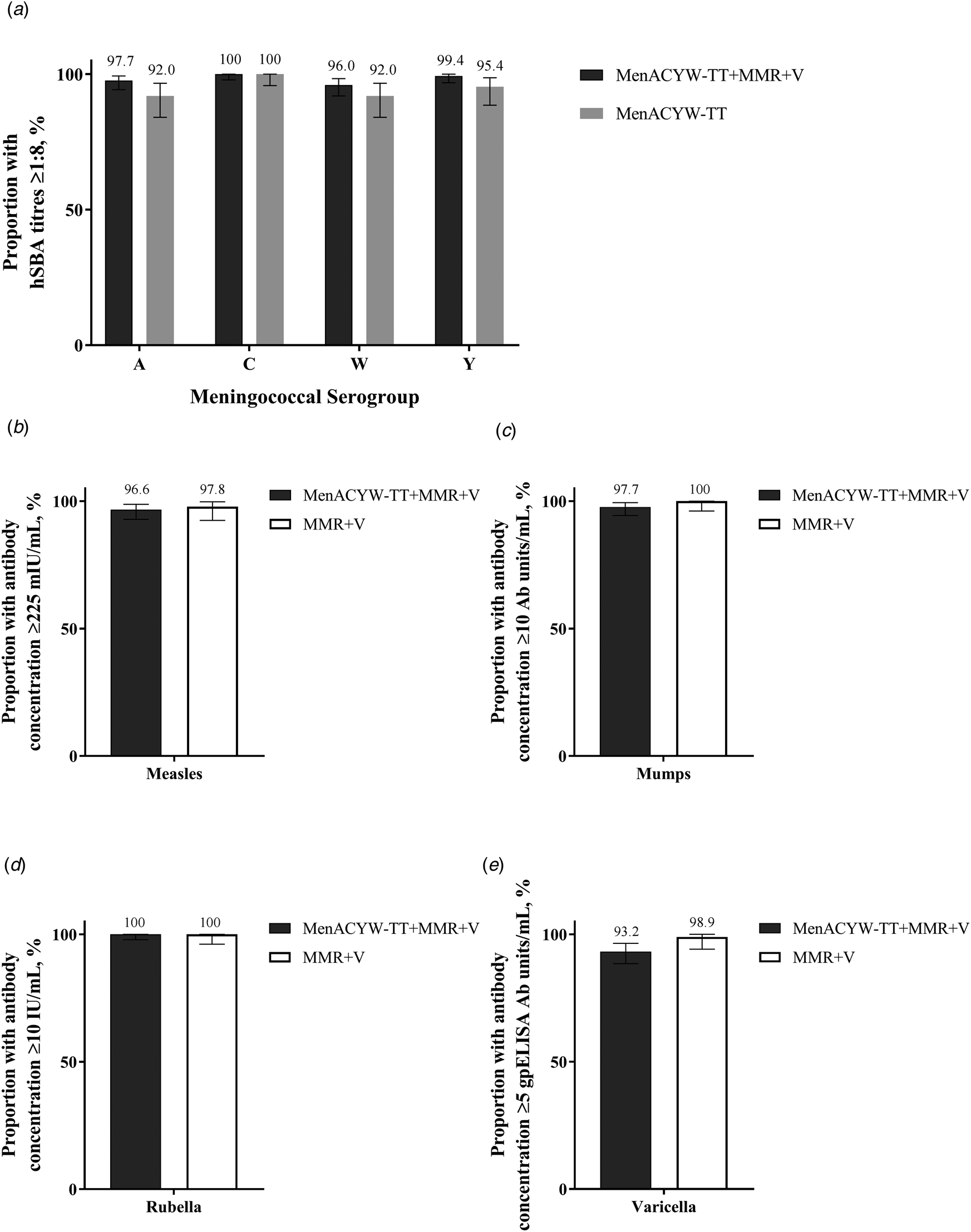
Immunogenicity and safety of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine administered concomitantly with other paediatric vaccines in toddlers: a phase III randomised study, Epidemiology & Infection

Immunogenicity and safety of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine administered concomitantly with other paediatric vaccines in toddlers: a phase III randomised study, Epidemiology & Infection

Randomized Clinical Trial To Evaluate the Immunogenicity of Quadrivalent Meningococcal Conjugate and Polysaccharide Vaccines in Adults in the United Kingdom

PDF) Meningococcal Quadrivalent Tetanus Toxoid Conjugate Vaccine (MenACWY-TT, Nimenrix™): A review of its Immunogenicity, Safety, Co-Administration, and Antibody Persistence

Meningococcal serogroups A, C, W-135, and Y tetanus toxoid conjugate v

Long-term antibody persistence after a booster dose of quadrivalent meningococcal ACWY-tetanus toxoid conjugate vaccine in healthy 5-year-old children - ScienceDirect

Safety and Immunogenicity of a Quadrivalent Meningococcal Co : The Pediatric Infectious Disease Journal

Vaccines, Free Full-Text

Full article: Immunogenicity and safety of a quadrivalent meningococcal conjugate vaccine (MenACYW-TT) administered as a booster to adults aged ≥59 years: A phase III randomized study
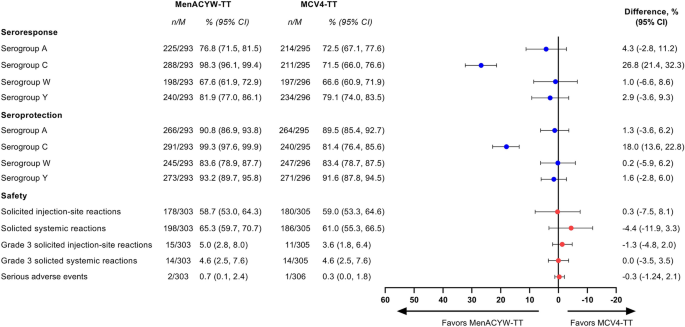
Structured Benefit-Risk Assessment of a New Quadrivalent Meningococcal Conjugate Vaccine (MenACYW-TT) in Individuals Ages 12 Months and Older

Sanofi Pasteur Announces FDA Approval of Menactra Meningococcal Conjugate Vaccine Indication for Infants







:watermark(cdn.texastribune.org/media/watermarks/2012.png,-0,30,0)/static.texastribune.org/media/videos/080912_Insider_Art.jpg)