Update on REMS-Required Testing During COVID-19 Pandemic - MPR
4.6 (308) · € 16.00 · En Stock
“The completion of some REMS-required laboratory testing or imaging studies may be difficult because patients suspected of having COVID-19 may be self-isolating and/or subject to quarantine,” said FDA Principal Deputy Commissioner Amy Abernethy, MD, PhD.

Coronavirus COVID-19 (SARS-CoV-2): Facts & Drug Discovery Updates (formerly 2019-nCoV)

U.S. allows 2 more over-the-counter COVID-19 home tests

Post-Thanksgiving COVID-19 bump in Minnesota, but RSV and flu hospitalizations down

Archived Webinars Readiness and Emergency Management for Schools Technical Assistance Center

FDA Issues New Guidance for REMS Testing During COVID-19 Pandemic

Coronavirus (COVID-19) Information
Medicina, Free Full-Text

Resources to Support Youth and Families During the COVID-19 Emergency

10-K
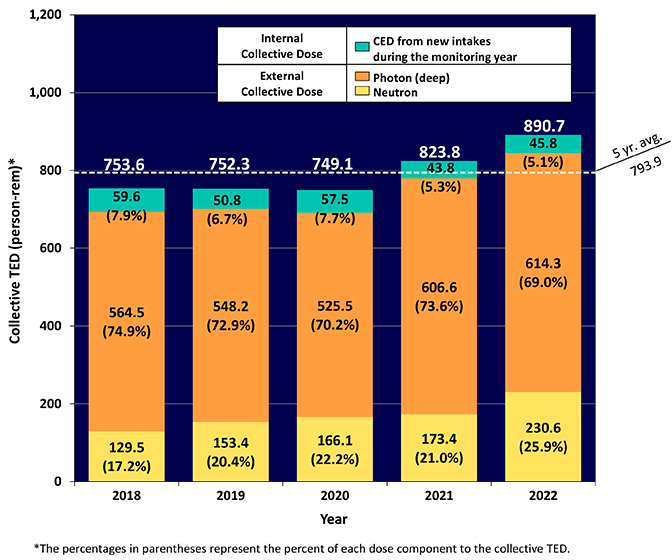
2021 Occupational Radiation Exposure Dashboard

Impact of the COVID-19 Pandemic on Emergency Department Visits — United States, January 1, 2019–May 30, 2020

Risk Evaluation and Mitigation Strategy programs: How they can be improved
COVID-19

iPLEDGE allows at-home pregnancy tests during pandemic - The Hospitalist











