- Accueil
- g heat
- Equal heat is gives to two objects A and B of mass 1 g. Temperature of A increases by 3oC and B by 5oC. Which object has specific heat? And by what
Equal heat is gives to two objects A and B of mass 1 g. Temperature of A increases by 3oC and B by 5oC. Which object has specific heat? And by what
4.8 (769) · € 23.50 · En Stock

Electric Potential Energy: Potential Difference

15.4 Carnot's Perfect Heat Engine: The Second Law of Thermodynamics Restated – College Physics: OpenStax

Final Temperature of Ice and Water Mixture - How Many Grams of Ice Will Melt?

Heat Revision MC Test, PDF, Electromagnetic Radiation
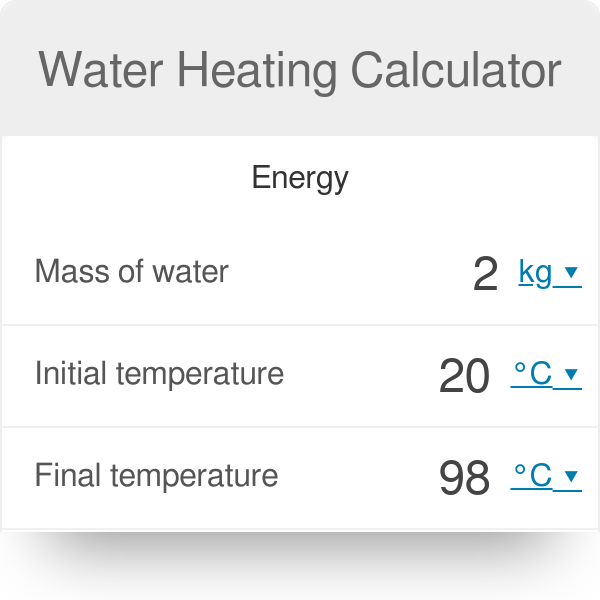
Water Heating Calculator

Thermal equilibrium

Thermal equilibrium

Specific Heat Capacity (比熱容量) - ppt download

Thermodynamics
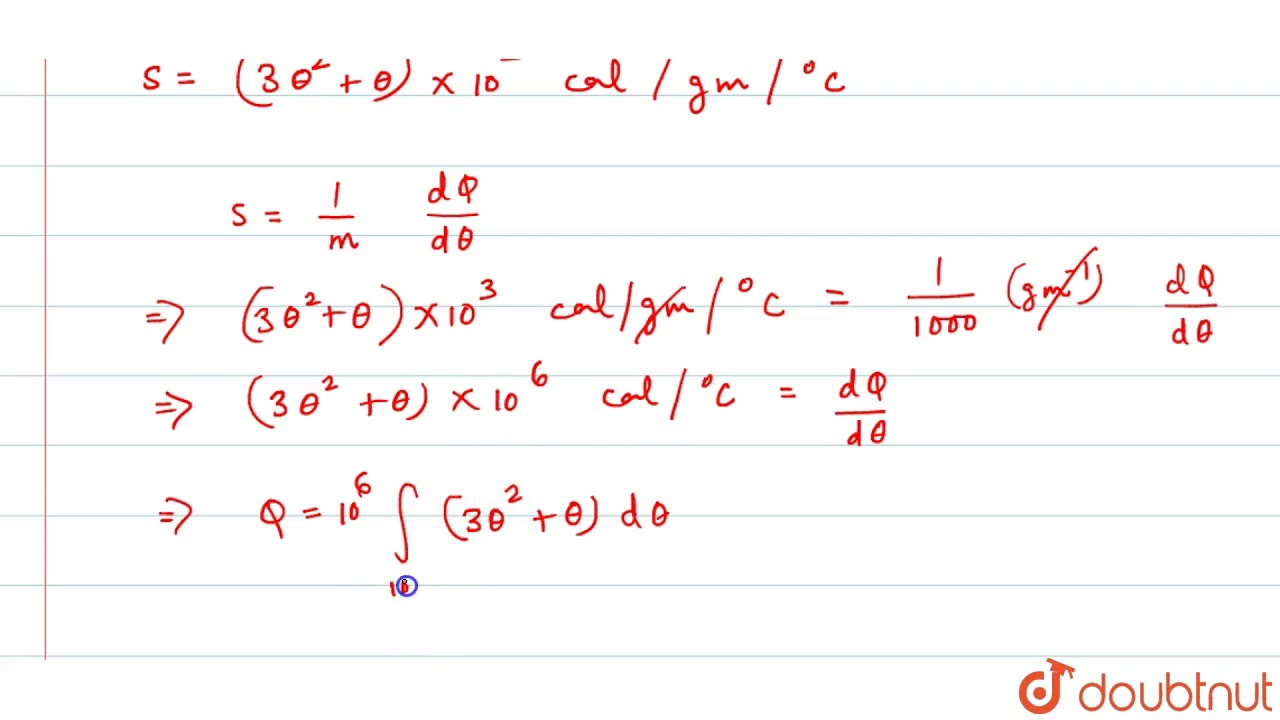
The specific heat of a substance varies as `(3 theta^(2) + theta) xx 10^(3) cal g^(-1) C^(-1)`.
Which contains more heat, 100. ml of water at 25 °C or a liter of water at 25 °C? What is the difference? - Quora
Tu pourrais aussi aimer





Proposer des recherches






© 2018-2024, thefforest.co.uk, Inc. ou ses affiliés
