Why Is Water a Polar Molecule?
5 (215) · € 19.99 · En Stock
Water is a polar molecule because the electrons are unevenly distributed. Since the molecule is polar, water is a polar solvent, also.
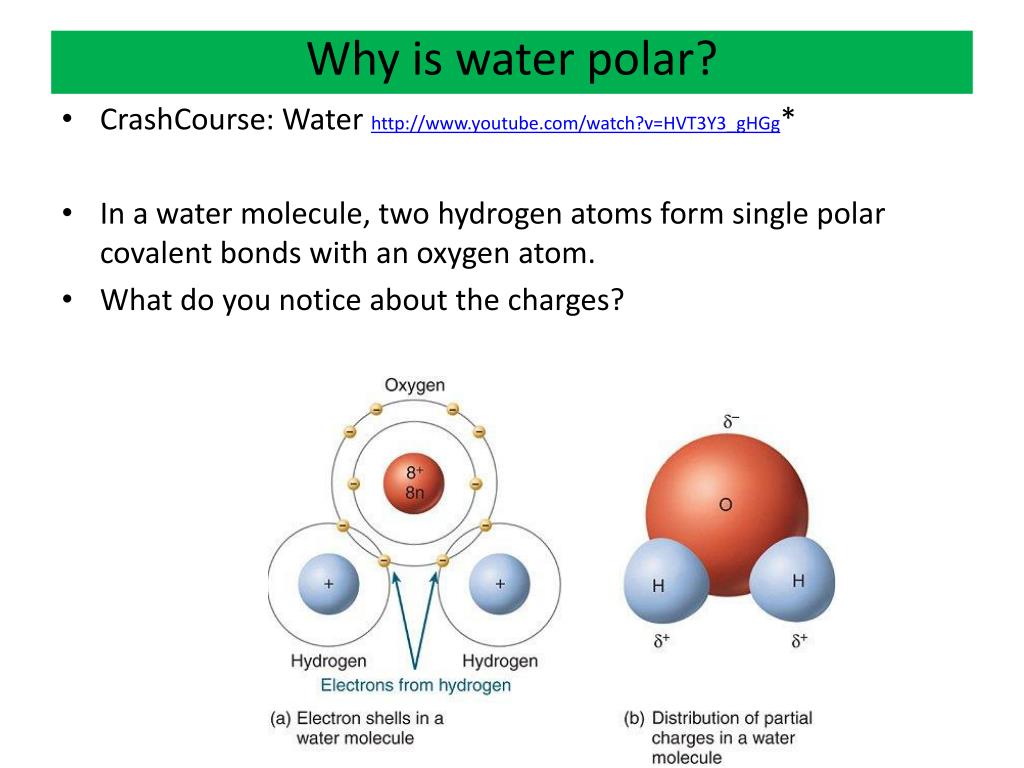
PPT - Ch 3: The Polarity of Water and Its Properties PowerPoint Presentation - ID:5635781

Solved Question 12 of 48 Water is a polar molecule because
Why does the bonding of oxygen and hydrogen result in a polar molecule and hydrogen bonds?
Water: Properties and Interactions

WHY WATER MOLECULE IS POLAR IN NATURE, NCERT, CHEMISTRY
:max_bytes(150000):strip_icc()/PolarConvalentBond-58a715be3df78c345b77b57d.jpg)
Why Is Water a Polar Molecule?

Polarity of Water (H2O) Molecule: Definition and Importance

Polar Molecule, Definition, Characteristics & Examples - Lesson

PPT - Water PowerPoint Presentation, free download - ID:1344926

Why is water (H2O) a polar molecule?
/oreillers-de-corps-eblouissement-cd-vierge.jpg.png)










:max_bytes(150000):strip_icc()/GettyImages-1041588324-5c3cf475c9e77c0001d63bca-5c3f692fc9e77c0001d9a10f.jpg)