SalivaDirect™ COVID-19 Testing Process < Pathology
4.5 (317) · € 35.99 · En Stock
Our quick and affordable saliva-based COVID-19 test developed by Yale scientists has received FDA Emergency Use Authorization. The Pathology Clinical Molecular
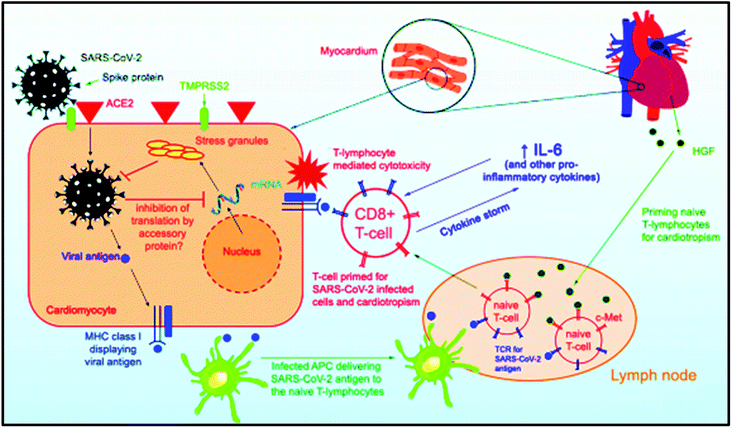
Insight into prognostics, diagnostics, and management strategies for SARS CoV-2 - RSC Advances (RSC Publishing) DOI:10.1039/D1RA07988C

Evaluation of saliva self-collection devices for SARS-CoV-2 diagnostics
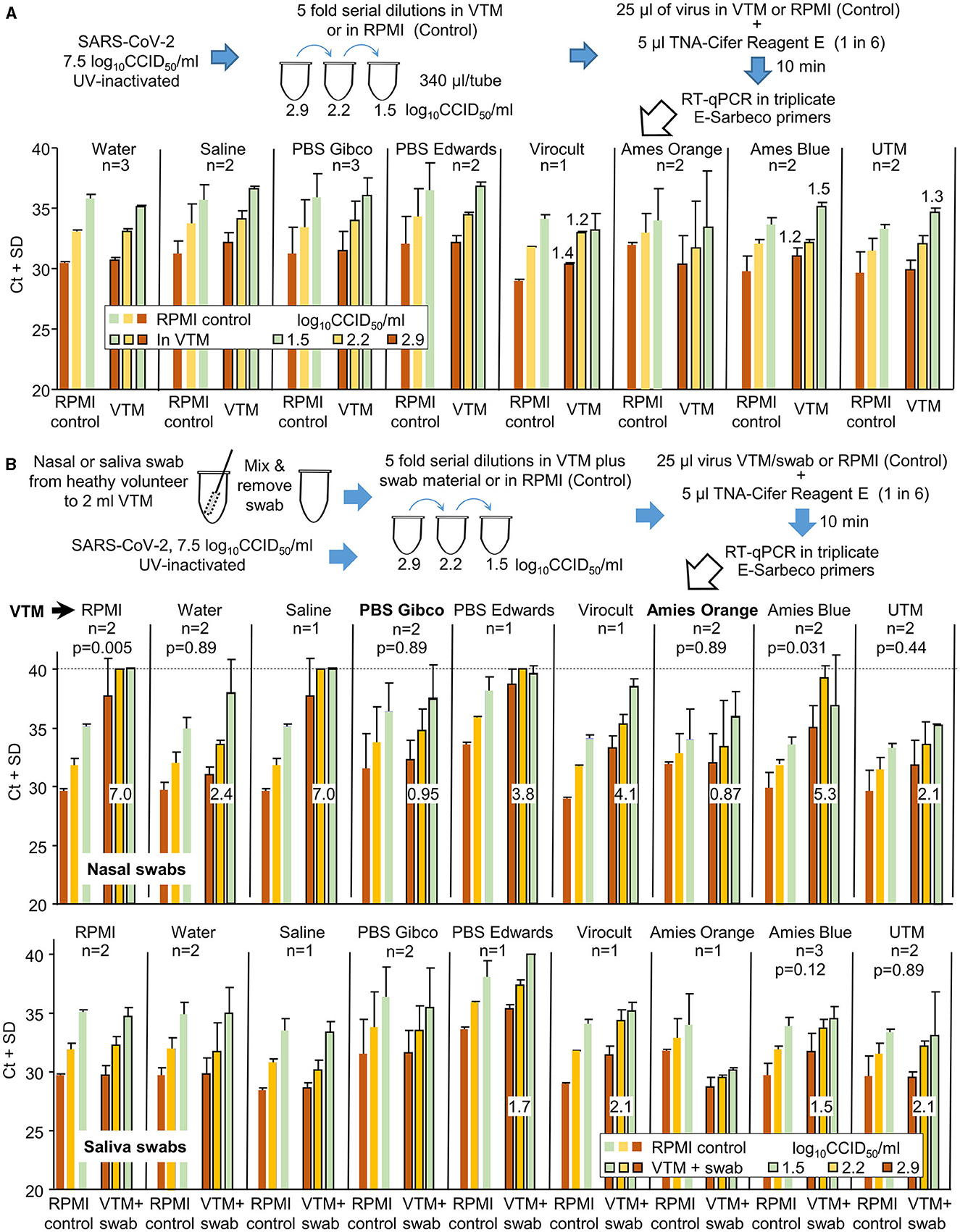
Frontiers Rapid inactivation and sample preparation for SARS-CoV-2 PCR-based diagnostics using TNA-Cifer Reagent E

Development and Implementation of a Simple and Rapid Extraction-Free Saliva SARS-CoV-2 RT-LAMP Workflow for Workplace Surveillance

COVID Test Direct Diagnostics
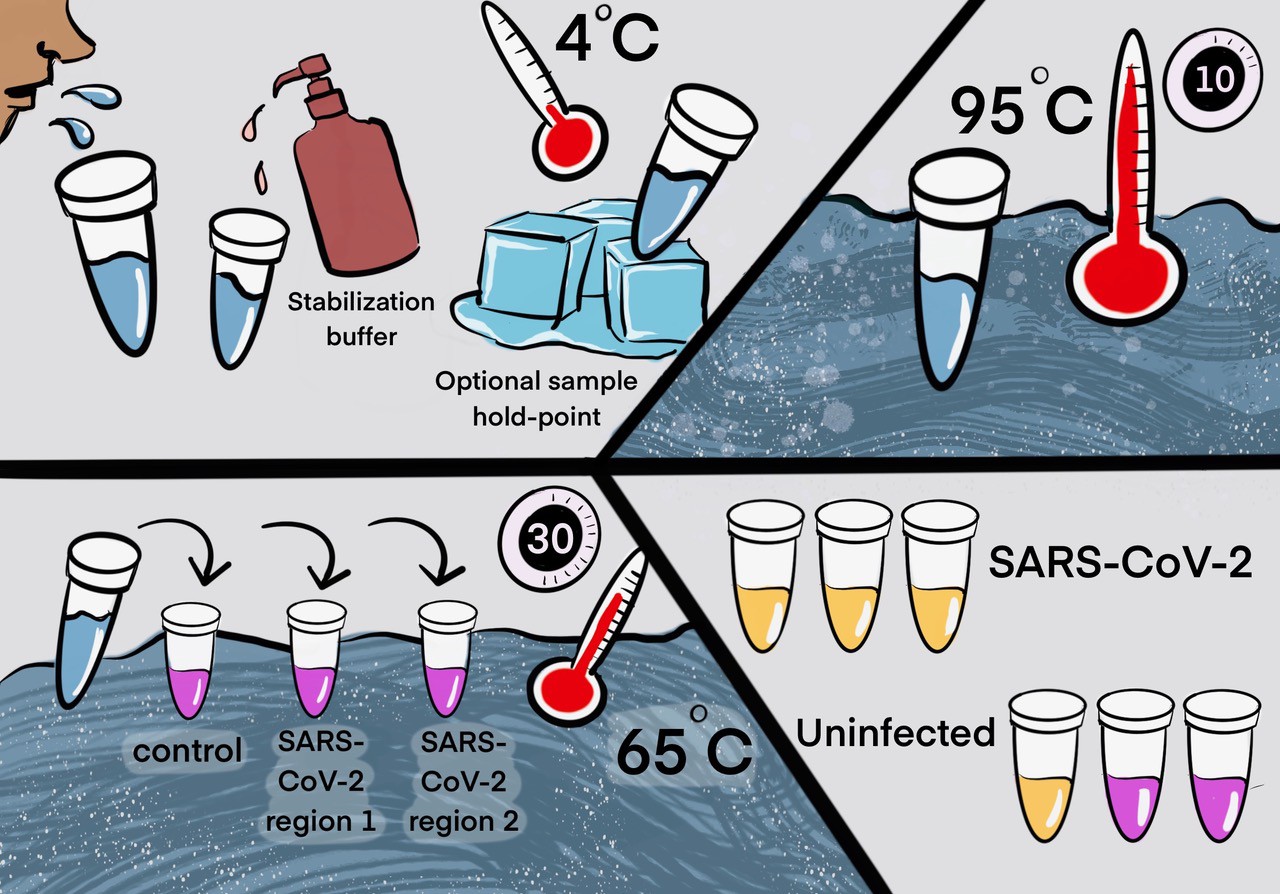
Saliva TwoStep for rapid detection of asymptomatic SARS-CoV-2 carriers

News SalivaDirect™

A place of pride for pathology labs < Yale School of Medicine
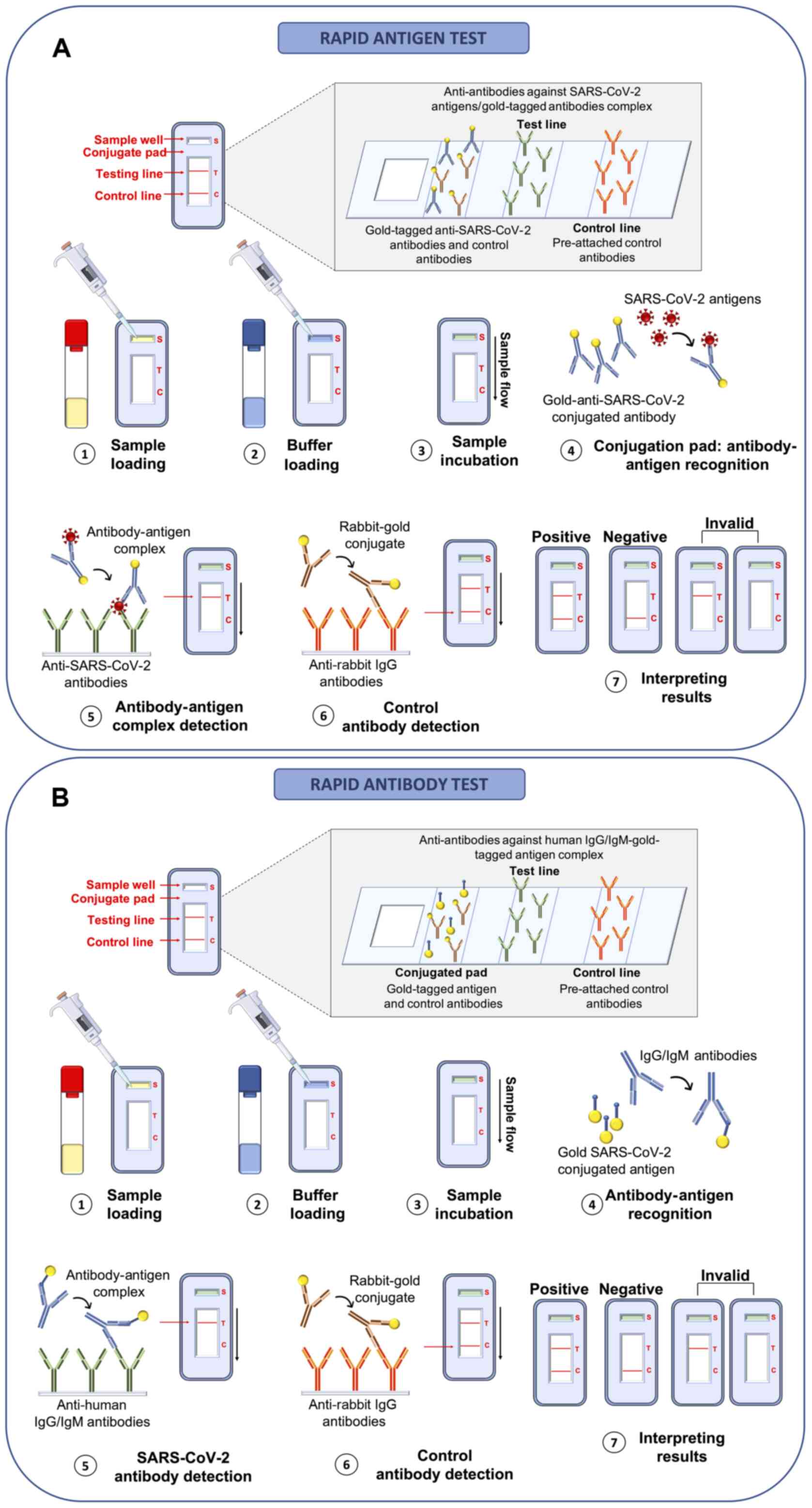
Current and innovative methods for the diagnosis of COVID‑19 infection (Review)
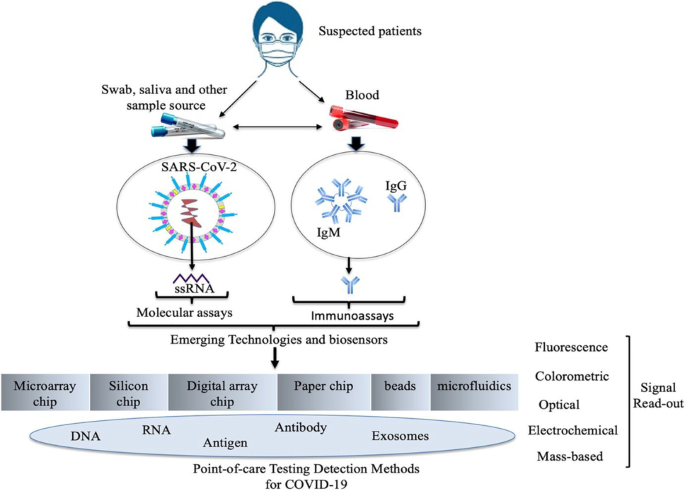
Diagnostic assay and technology advancement for detecting SARS-CoV-2 infections causing the COVID-19 pandemic
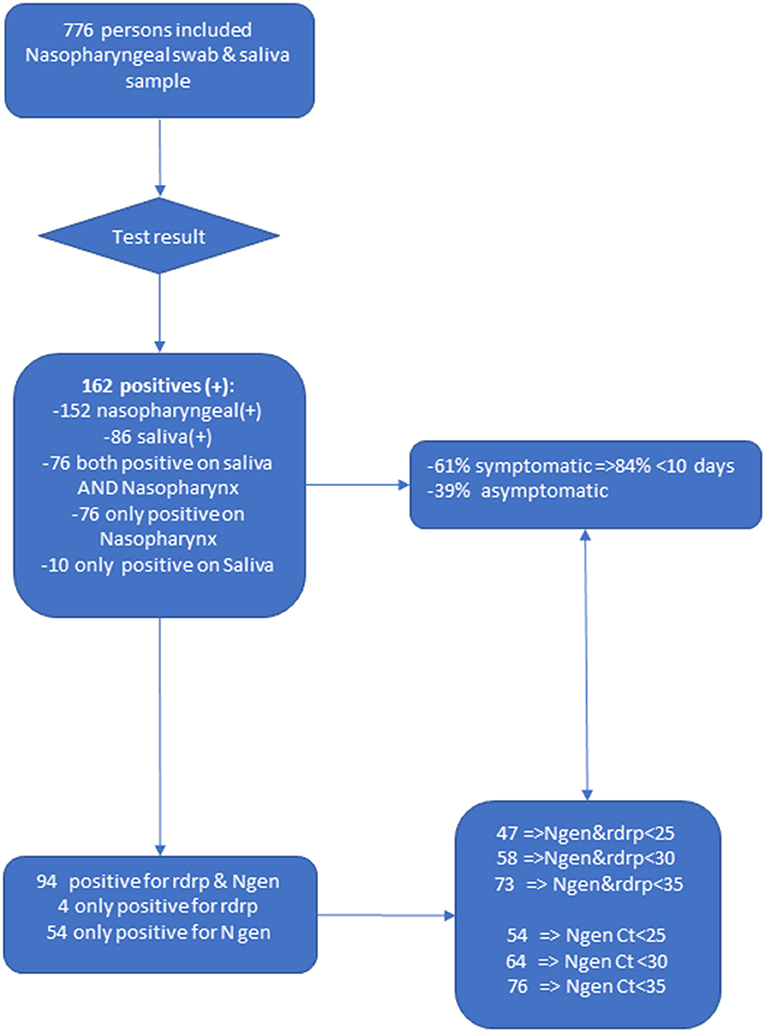
Frontiers Prospective Comparison of Saliva and Nasopharyngeal Swab Sampling for Mass Screening for COVID-19
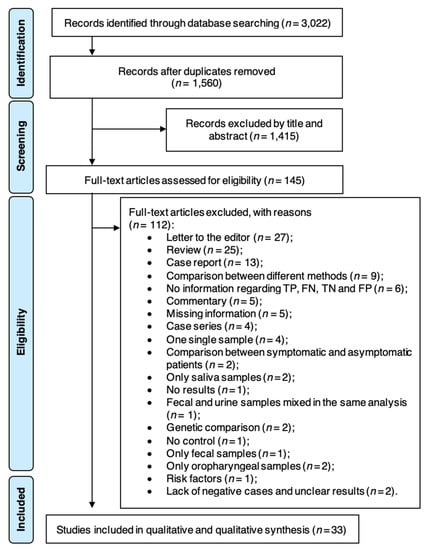
Diagnostics, Free Full-Text




)