The Submission Dossier Regulatory Affairs in Latin America
4.6 (407) · € 22.50 · En Stock
The submission dossier is the packet of documents that are to be submitted to a health authority for registration of a product, or for other life-cycle maintenance activities, such as renewal of registration or CMC variations. The requirements vary very widely from country to country, but in general a dossier contains administrative documents, (such as…

Regulatory Affairs in Latin America

Regulatory Affairs

Drugs, Biologics and Medical Devices Regulatory Affairs Course - Royed Training

Regulatory Affairs - Pharmaceutical Technology

Latin America: Understanding Regulatory Compliance Requirements Across the Life Science Industry by ComplianceOnline - Issuu

PDF) Recent drug regulatory affair and CTD module progress review for submission of pharmaceuticals product

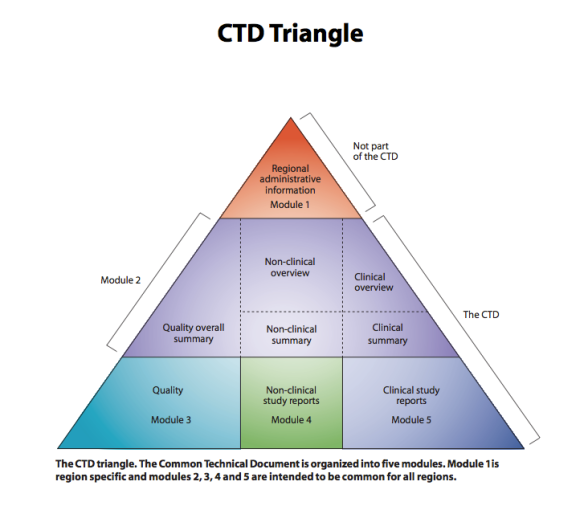
Brazil's Anvisa Organization of the Common Technical Document (CTD)

External Drivers for Regulatory Outsourcing
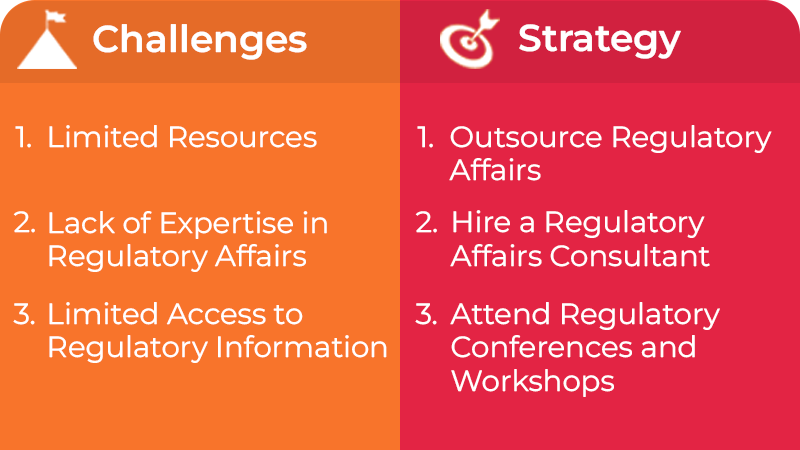
Regulatory submission challenges faced by small and midsize organizations