Difference between Strong and Weak Base - with Examples [in Table]
4.6 (696) · € 34.50 · En Stock
Strong BaseWeak BaseThey get completely ionized (split up into ions) in water and produce large amounts of hydroxide ions.These only get partially ionized (split up into ions) in water and produce less amount of hydroxide ions.pH value is close to 14 but smaller than it.pH value is closer to 7 but

Weak Acids and Weak Bases
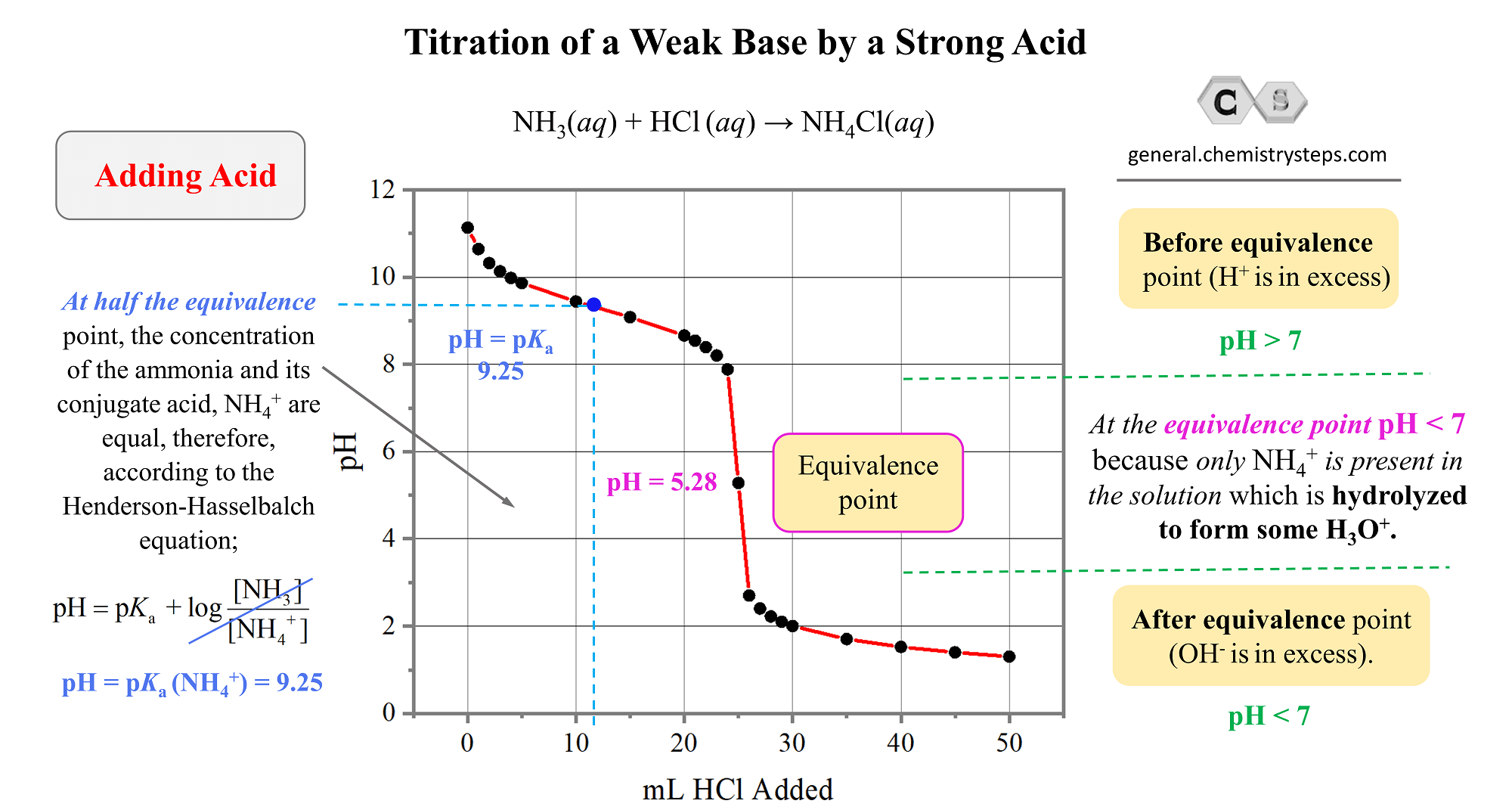
Titration of a Weak Base by a Strong Acid - Chemistry Steps
Acid Strength - Definition, Acid Strength Order, Chart, Trends & Factors Affecting Acid Strength
Classifying Electrolytes

Titration curves & equivalence point (article)
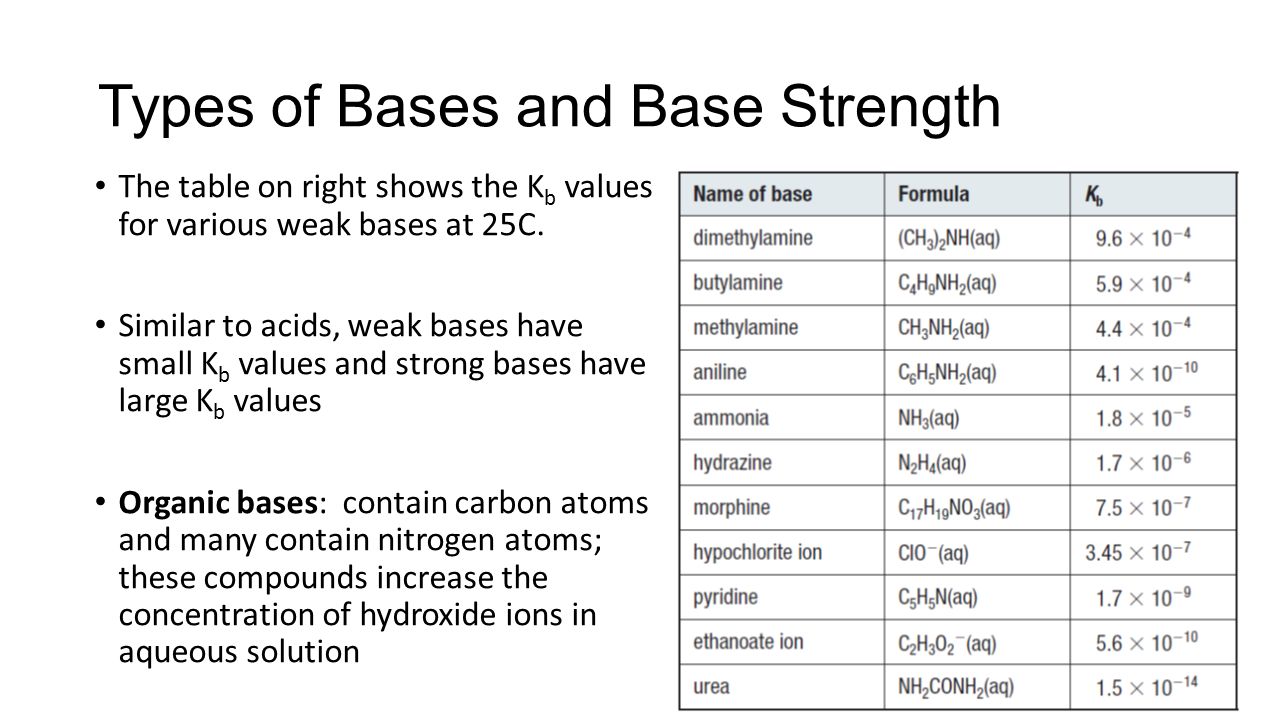
Strong and Weak Acids and Bases - ppt video online download
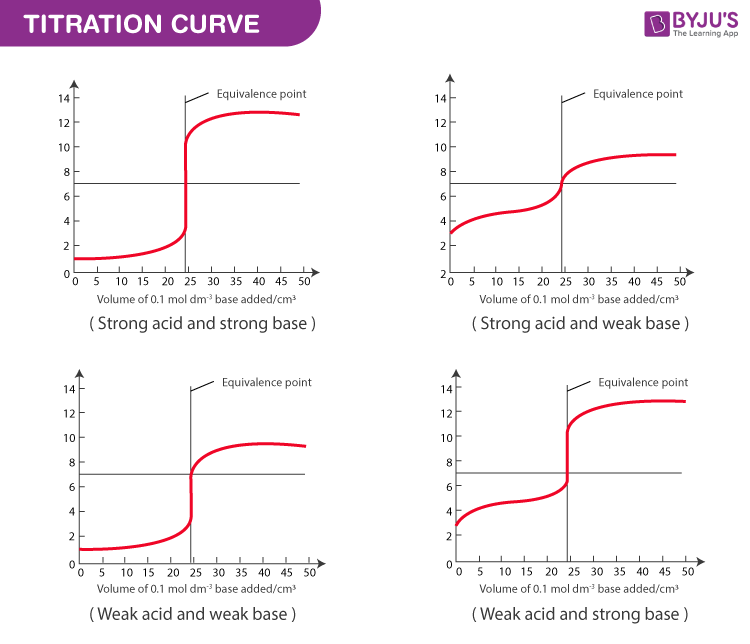
Acid Base Titration - Titration Curves, Equivalence Point & Indicators of Acid Base Titration

How To Memorize The Strong Acids and Strong Bases

Acid & Base Properties of Water, Overview & pH Measurement - Video & Lesson Transcript






![Difference between Strong and Weak Base - with Examples [in Table]](https://d1avenlh0i1xmr.cloudfront.net/0e323ff3-079c-4d5e-b21f-0f4c25082521/differences-between-strong-and-weak-bases-01.jpg)


:max_bytes(150000):strip_icc()/Sodium-hydroxide-58fa465d5f9b581d59f017e3.jpg)


