Xpert® Xpress SARS-CoV-2 - FDA Emergency Use Authorization
5 (278) · € 18.99 · En Stock
Xpert® Xpress SARS-CoV-2/Flu/RSV received Emergency Use Authorization from the US FDA to support the global fight against COVID-19, with rapid detection of the current coronavirus SARS-CoV-2.

Xpert® Xpress SARS-CoV-2 - FDA Emergency Use Authorization

FDA Approves 45-Minute Point-of-Care COVID-19 Diagnostic Test

Federal Register :: Revocation of Three Authorizations of Emergency Use of In Vitro Diagnostic Devices for Detection and/or Diagnosis of COVID-19; Availability

Cepheid Receives Emergency Use Authorization For SARS-CoV-2, Flu A, Flu B, and RSV Combination Test

Medical Countermeasures USA Public Health Security

Four-In-One-Test for Respiratory Virus Season
Fast, portable tests come online to curb coronavirus pandemic
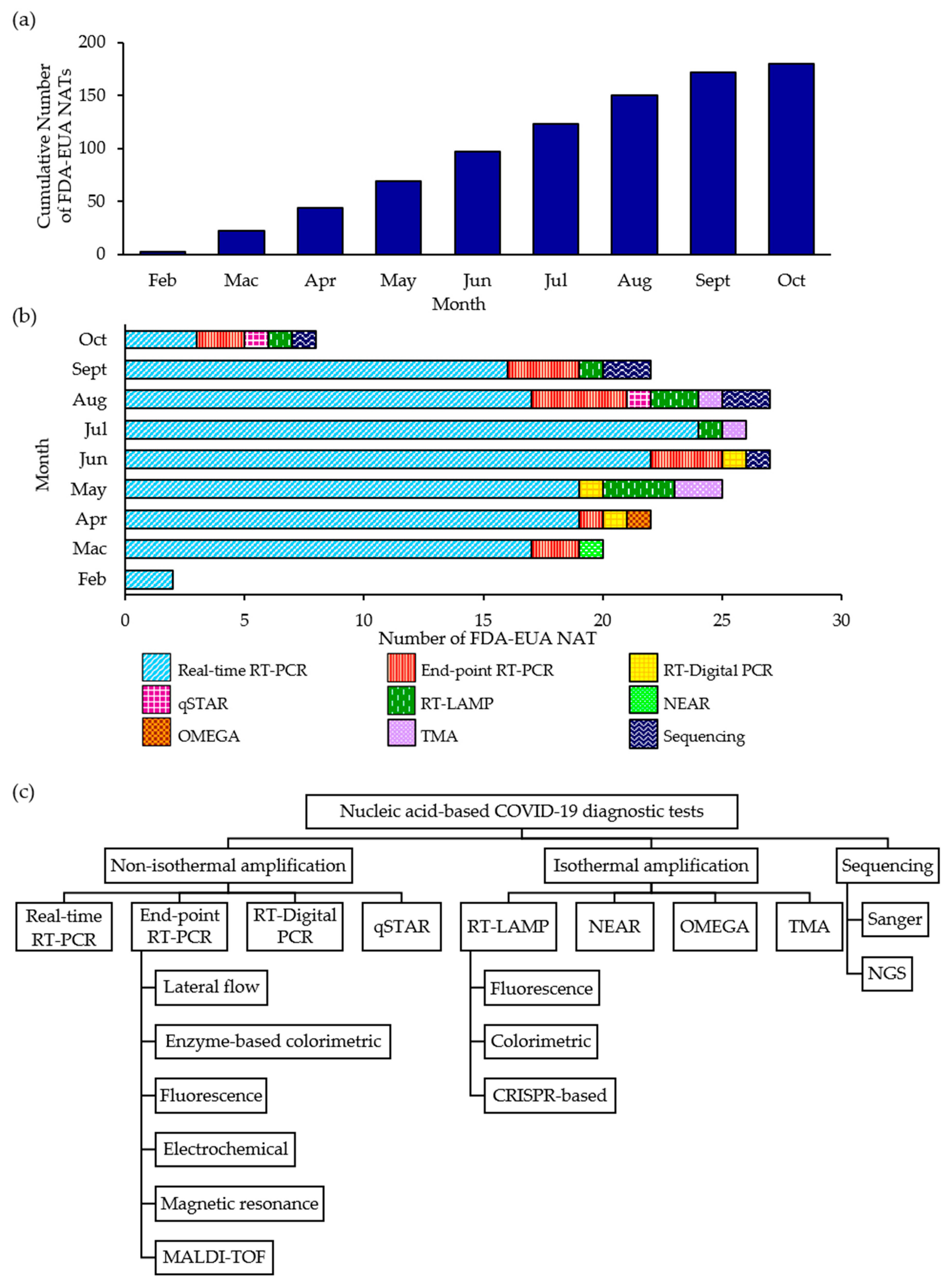
Diagnostics, Free Full-Text

New COVID-19 Rapid Diagnostic Approved On 'GeneXpert' TB Platform; Could Pave Way For More Testing In Low- & Middle-Income Countries - Health Policy Watch
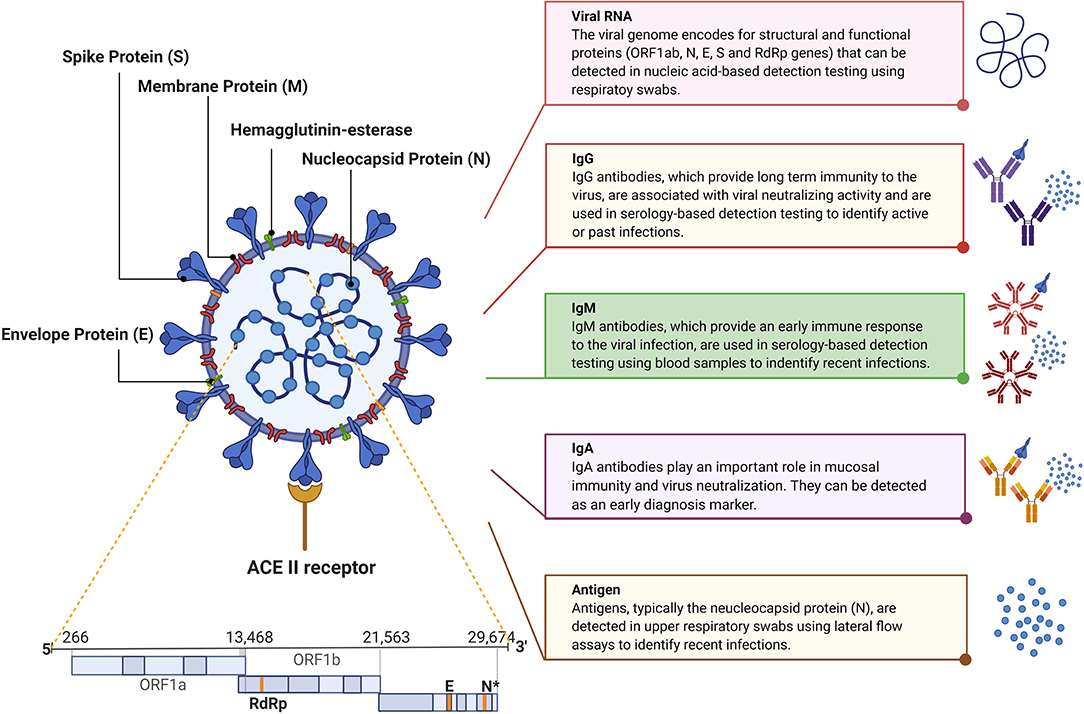
Frontiers COVID-19 in-vitro Diagnostics: State-of-the-Art and Challenges for Rapid, Scalable, and High-Accuracy Screening
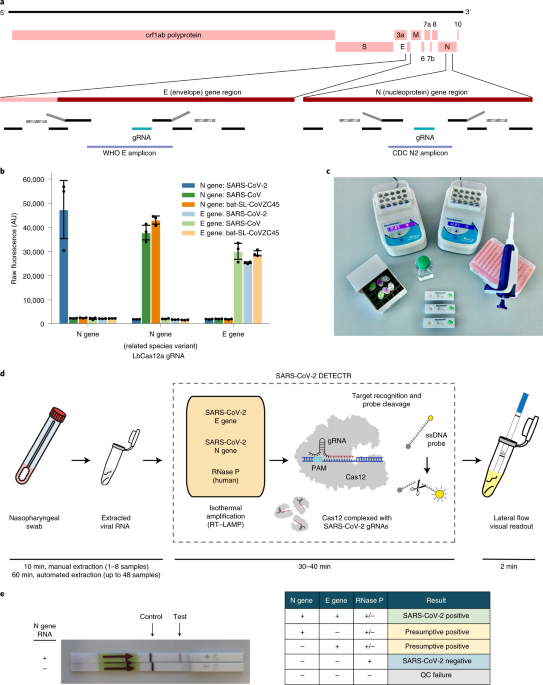
CRISPR–Cas12-based detection of SARS-CoV-2

Cepheid on X: For the first time, our upcoming educational webinar about improving SARS-CoV-2 testing will be available in multiple languages. Join us! Sign up at / X
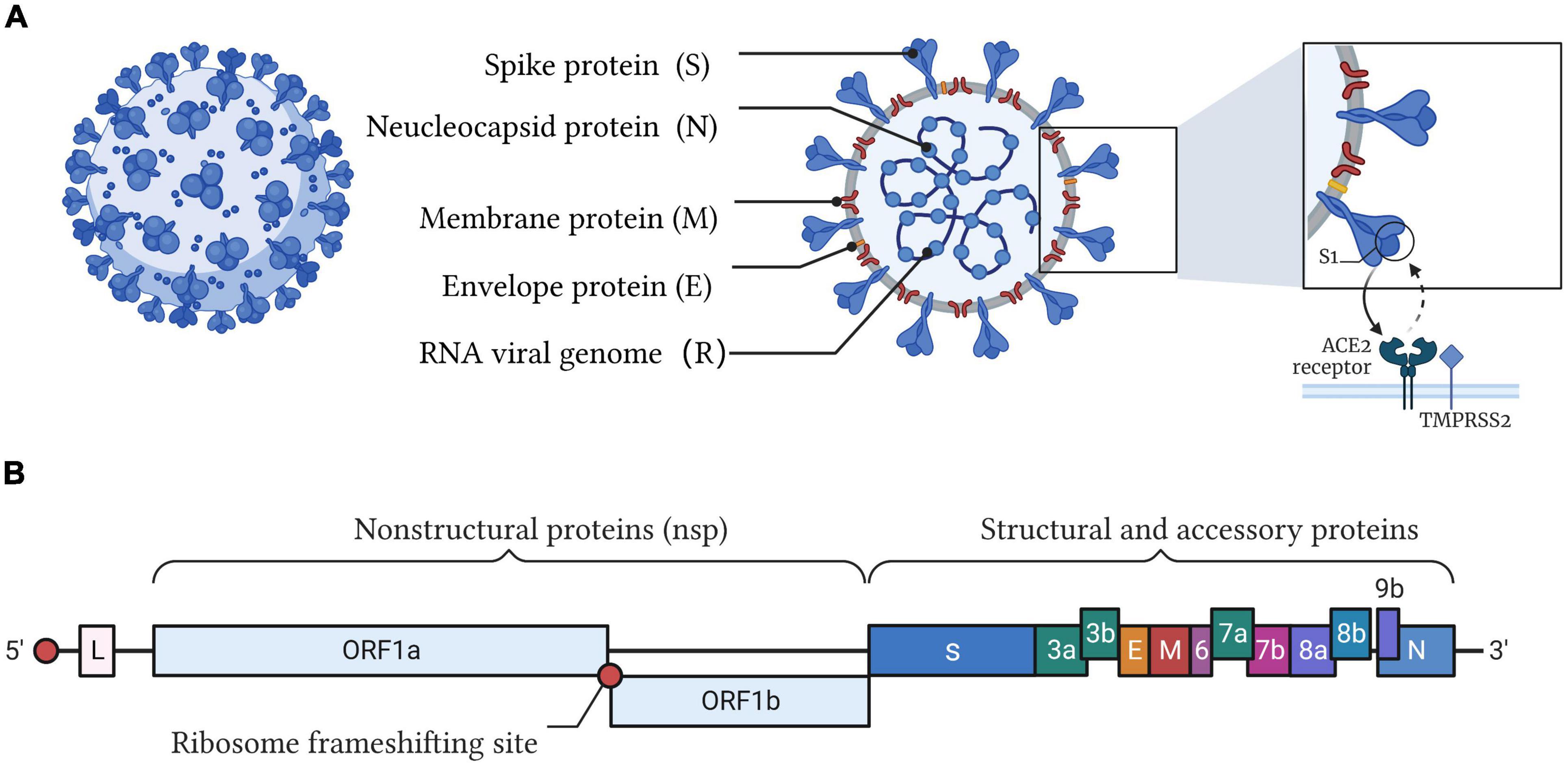
Frontiers Nucleic acid testing of SARS-CoV-2: A review of current methods, challenges, and prospects








.png)