Avoid Launch Delays By Planning For An FDA-Required REMS Risk
4.9 (786) · € 20.50 · En Stock
lt;p>Picture this: The FDA accepts a manufacturer's NDA, and the manufacturer plans for its impending launch. But shortly before the anticipated approval, the FDA notifies the manufacturer that a Risk Evaluation and Mitigation Strategy (REMS) program is required to market the product. Now what?</p>

Understanding Risk Evaluation and Mitigation Strategy (REMS)

A Look Back at Risk Evaluation and Mitigation Strategies at the Food and Drug Administration in 2020: Year in Review - Food and Drug Law Institute (FDLI)

Mycophenolate Fetal Toxicity and Risk Evaluation and Mitigation Strategies - ScienceDirect

Drug Safety and the Cost of Monitoring: The Role of REMS in Risk Management - Mark Slomiany, Rema Bitar, Sarah Kruse, Sarah Jeffers, Kenneth Berkowitz, Mahmud Hassan, 2015

REGULATORY AND OTHER PITFALLS IN DRUG DEVELOPMENT

REMS in Oncology

ny20009765x5_ex99-1img01.jpg
These highlights do not include all the information needed to use FILSPARITM safely and effectively. See full prescribing information for FILSPARITM. FILSPARITM (sparsentan) tablets, for oral useInitial U.S. Approval: 2023

Jazz Pharmaceuticals plc 2021 Directors' Report and Financial Statements

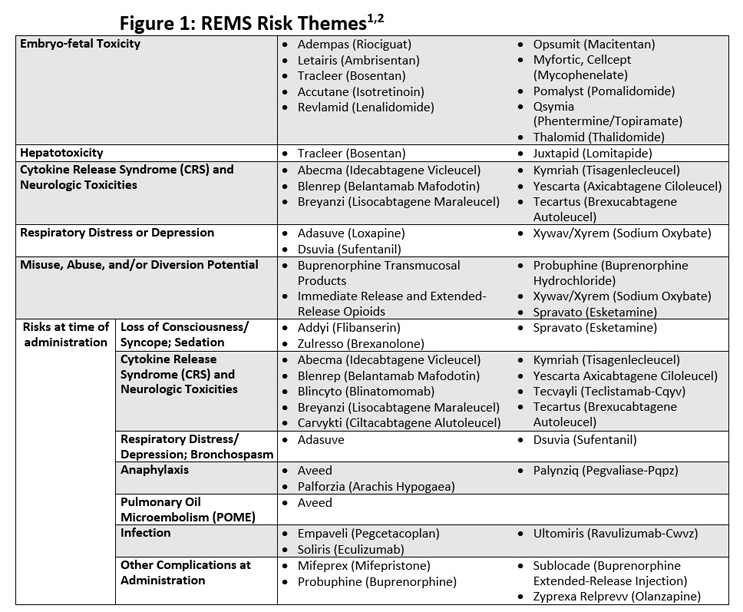
Figure A4. Text extracted from REMS materials to illustrate content
Avoid Launch Delays By Planning For An FDA-Required REMS Risk Evaluation and Mitigation Strategy

Post-marketing authorisation safety and efficacy surveillance of advanced therapy medicinal products in Brazil, the European Union, the United States and Japan - Cytotherapy

Understanding The FDA's Current Focus On REMS

REMS Modernization Can't Wait A Call to Action