2024-2025 Regulatory Agenda Has Been Approved by the Brazilian FDA - Lexology
4.7 (644) · € 30.00 · En Stock
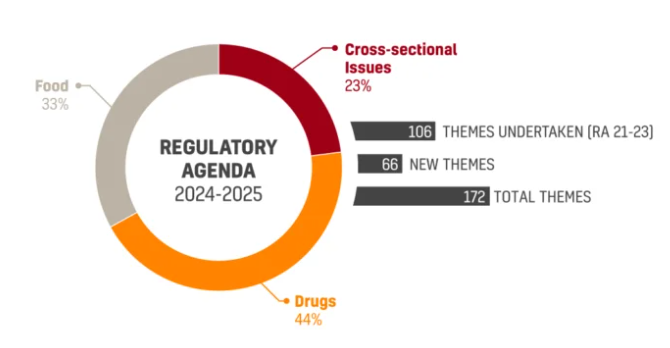
ANVISA’s new Regulatory Agenda 2024-2025 (RA 24/25) was approved and published on the Official Gazette on December 18th. The Joint Ordinance No. 1…

2023 - 2025 Action Plan for the Brazilian Intellectual Property

6th Neuroimmunology Drug Development Summit

Abstract Submissions - WORLDSymposium
.jpg?rev=a681631fe4954c1a93b45372334bdc74&w=790&hash=D2DEFDB12951E171451EEE9624AC2CA9)
Celltrion's Vegzelma Becomes Fourth US-Approved Bevacizumab

Advertising law, year in review

In review: the life sciences regulatory regime in Brazil - Lexology

Abstracts – Ever Congress

Snapshot: procedure for design registration in Brazil - Lexology

Anvisa publishes new Regulatory Agenda for 2024-2025 — Food

February 7, 2023: FDA Issues Draft Guidance for Use of Real-World
Tu pourrais aussi aimer





Proposer des recherches






© 2018-2024, thefforest.co.uk, Inc. ou ses affiliés